Adderall and Ketamine: Can You Mix Adderall and Ketamine?
Mixing Adderall and ketamine creates overlapping cardiovascular and psychiatric risks that can be dangerous.
Both drugs activate the sympathetic nervous system, raising blood pressure and heart rate, while ketamine’s dissociative effects can mask or worsen stimulant-related agitation.
In medically supervised settings, protocols exist to manage these risks, but recreational mixing is high-risk and should be avoided.
This article explains the interaction between Adderall and ketamine, the short-term side effects of combining them, and what you need to know to stay safe.
What Happens When You Mix Adderall and Ketamine?
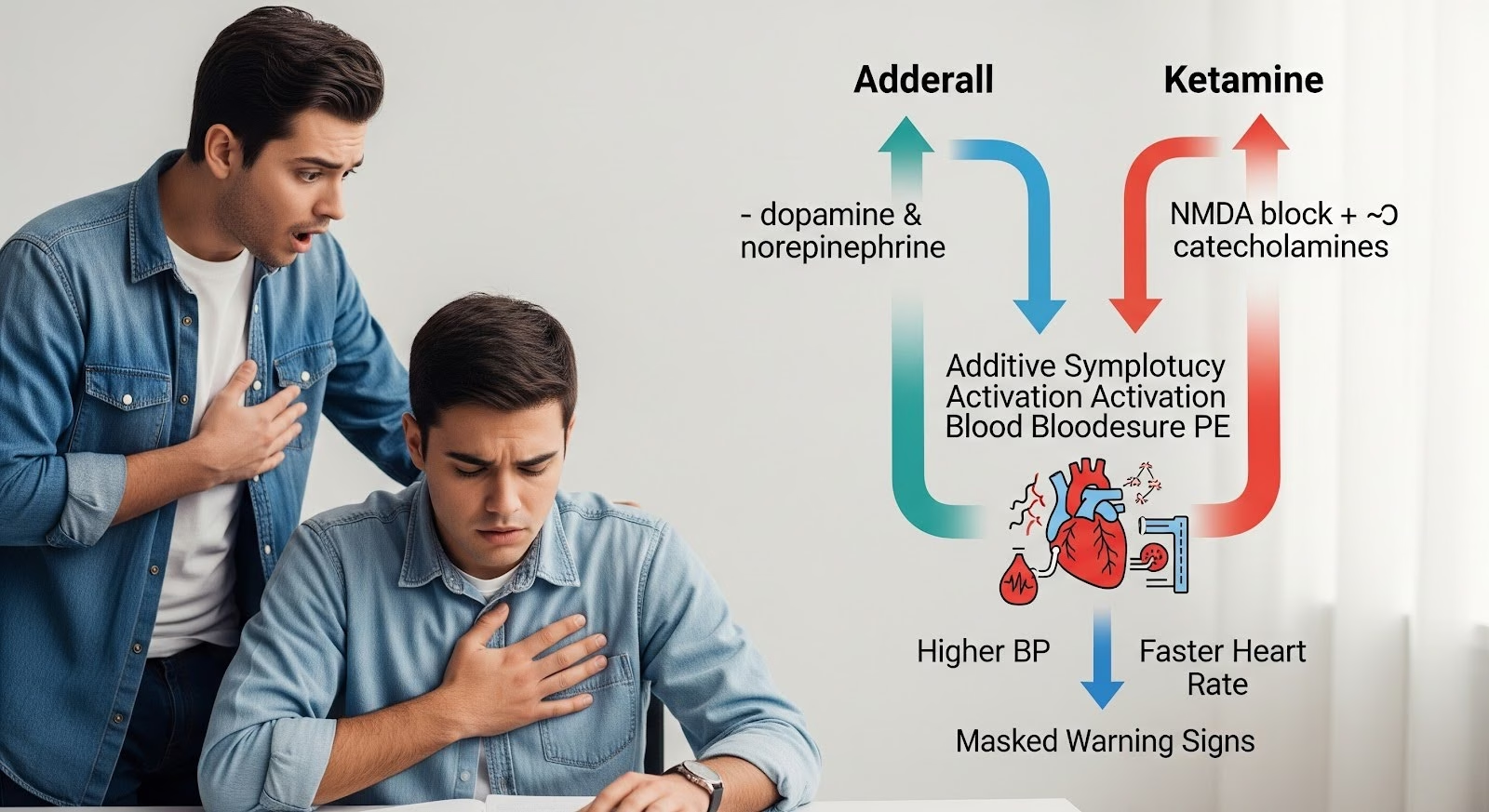
Adderall (mixed amphetamine salts) and ketamine both influence the body’s catecholamine system, which controls heart rate, blood pressure, and arousal.
Adderall increases dopamine and norepinephrine by promoting their release and blocking their reuptake. Ketamine, while primarily an NMDA receptor antagonist, also inhibits catecholamine reuptake and stimulates catecholamine release in the peripheral nervous system.
When combined, these mechanisms create additive sympathetic activation, meaning the cardiovascular effects stack on top of each other.
Blood pressure and heart rate rise more than with either drug alone. This interaction is mechanistically plausible and clinically recognized in emergency medicine and anesthesia practice.
In therapeutic contexts, esketamine (the S-enantiomer of ketamine, marketed as Spravato) is sometimes prescribed to patients already taking stimulants for ADHD.
The Canadian Product Monograph for Spravato requires monitored administration with blood pressure checks before and after dosing, acknowledging the hemodynamic risks of co-exposure.
Cardiovascular Risks of Combining Adderall and Ketamine
The primary short-term danger of mixing Adderall and ketamine is cardiovascular strain. Both drugs raise blood pressure and heart rate through overlapping pathways.
Amphetamine-type stimulants cause peripheral vasoconstriction and cardiac stimulation. At therapeutic doses, these effects are usually mild, but at higher doses or in people with underlying heart conditions, they can trigger hypertension, arrhythmias, or even stroke.
Ketamine similarly increases sympathetic tone, with blood pressure and heart rate elevations commonly observed during administration.
When both drugs are present, the pressor effects add together. A person on a moderate dose of Adderall who then uses ketamine may experience a sharp spike in blood pressure that would not occur with either drug alone.
This risk is highest in people with hypertension, structural heart disease, or cerebrovascular disease.
Emergency department data support this concern. Ketamine is used to control severe agitation, including in stimulant-intoxicated patients, but clinical policy from the American College of Emergency Physicians recommends benzodiazepine-antipsychotic combinations as first-line treatment for agitation, reserving ketamine for situations where immediate safety is at risk.
This reflects awareness of ketamine’s hemodynamic liabilities, especially in sympathomimetic states.
Anesthesia safety reviews note that patients with methamphetamine use disorder can develop refractory hypertension during procedures.
While these reviews focus on illicit stimulants, the pharmacology applies to prescription amphetamines like Adderall. Anesthesia professionals are advised to anticipate difficult blood pressure control and have antihypertensive strategies ready.
Key Cardiovascular Effects
- Additive increases in blood pressure and heart rate
- Elevated myocardial oxygen demand
- Potential for arrhythmias in susceptible individuals
- Risk of hypertensive crisis at high doses or in vulnerable patients
Psychiatric and Neurological Risks
Beyond cardiovascular effects, the ketamine and Adderall interaction carries psychiatric risks. Ketamine causes dissociation, perceptual changes, and psychotomimetic experiences.
Adderall increases arousal, anxiety, and at high doses, can trigger psychosis. Together, these effects can create acute psychiatric instability.
Pharmacovigilance data from the FDA Adverse Event Reporting System (FAERS) show signals for dissociation, sedation, suicidal ideation, and completed suicide with esketamine. While spontaneous reports cannot prove causation, they highlight the need for careful monitoring.
Case reports describe paradoxical worsening of depression and emergent suicidal ideation in two patients receiving intranasal esketamine for treatment-resistant depression. Both improved after discontinuation.
These cases are rare but underscore the importance of mood monitoring during ketamine treatment, especially in patients on stimulants who may already have comorbid anxiety or depressive symptoms.
Stimulant misuse is common among people prescribed ADHD medications. A 2025 systematic review found that 22.6% of individuals prescribed stimulants reported past-year misuse, and 18.2% reported past-year diversion.
Risk factors included being prescribed an amphetamine-based stimulant, comorbid depression or anxiety, and perceiving misuse as low-risk. This overlap between stimulant misuse and psychiatric comorbidity increases the likelihood of uncontrolled co-use with ketamine.
Ketamine is also documented as an adulterant in stimulant supplies, including methamphetamine and MDMA. People who use stimulants recreationally may unknowingly be exposed to ketamine, compounding cardiovascular and neurological risks.
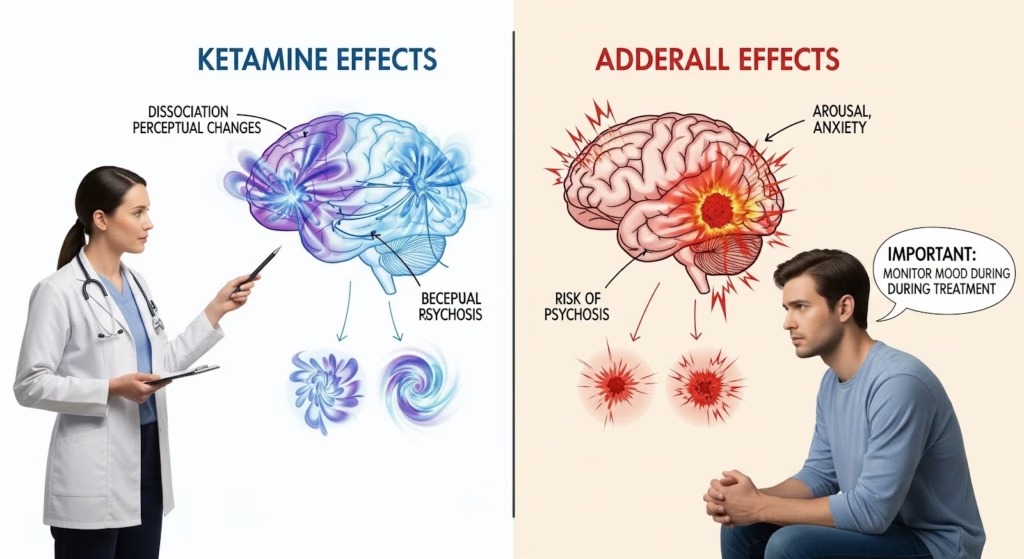
Short-Term Side Effects of Mixing Adderall and Ketamine
The table below summarizes the expected short-term adverse effects when Adderall and ketamine are combined, based on mechanistic pharmacology, clinical labeling, and emergency medicine practice.
| Effect | Mechanism | Clinical Significance |
| Hypertension | Additive catecholamine release and reuptake inhibition | Can trigger stroke or myocardial infarction in vulnerable patients |
| Tachycardia | Sympathetic activation from both drugs | Increases myocardial oxygen demand; risk of arrhythmia |
| Dissociation | Ketamine NMDA antagonism | Can be distressing; may mask or worsen stimulant-related agitation |
| Anxiety/agitation | Stimulant arousal plus ketamine psychotomimetic effects | Heightened psychiatric instability; risk of panic or psychosis |
| Nausea/vomiting | Common with ketamine | Discomfort; aspiration risk if sedated |
| Emergence reactions | Ketamine-related perceptual disturbances | Occur in 10–20% of ketamine users; managed with benzodiazepines |
These effects are most pronounced when doses are high, when mixing occurs in unmonitored settings, or when individuals have underlying cardiovascular or psychiatric vulnerabilities.
Medical Use: Esketamine and Stimulant Co-Prescribing
In clinical practice, some patients receiving esketamine for treatment-resistant depression are also prescribed stimulants for ADHD. This scenario is explicitly anticipated in esketamine labeling and risk mitigation programs.
The Spravato Canadian Product Monograph requires administration in a controlled setting with pre-dose blood pressure assessment and at least two hours of post-dose monitoring. Patients must not drive on the day of treatment due to sedation and dissociation risks.
While labeling does not contraindicate stimulant co-prescribing, the additive pressor risk necessitates careful management. Best practices include:
- Holding or reducing the stimulant dose on esketamine treatment days
- Checking baseline blood pressure and heart rate before dosing
- Monitoring vital signs at intervals after administration
- Having antihypertensive and anxiolytic medications available
- Screening for mood worsening or suicidal ideation at each session
A practical approach is to ask patients to skip their morning Adderall dose on esketamine days and resume it later in the day only after vital signs have stabilized and dissociative effects have resolved. This minimizes the overlap of peak drug effects.
Patients with hypertension, arrhythmia, or structural heart disease should undergo cardiovascular evaluation before starting esketamine, especially if they are on stimulants. Cardiology consultation may be warranted for high-risk individuals.
Recreational Mixing: Why It’s Dangerous?
Outside medical settings, mixing Adderall and ketamine is high-risk. Recreational use lacks the safeguards of clinical administration: no pre-dose screening, no vital sign monitoring, no emergency protocols, and often unpredictable drug purity and dosing.
Ketamine is sometimes used in nightlife settings to “temper the crash” after stimulant binges. However, this practice does not reduce cardiovascular strain.
In fact, the combination can produce a confusing subjective state where dissociation masks the body’s warning signs of sympathetic overload, such as chest pain or severe headache.
Emergency department networks in Europe document frequent stimulant-related toxicity presentations involving polysubstance use, with agitation, hyperthermia, and need for sedation or intubation.
While these reports focus on illicit stimulants like methamphetamine and MDMA, the pharmacology applies to Adderall.
Harm reduction messaging should emphasize:
- Avoid mixing stimulants and ketamine due to additive cardiovascular and psychiatric risks
- Be aware that ketamine may be present as an adulterant in stimulant supplies
- If co-use occurs, use the lowest doses possible, avoid hot or crowded environments, stay hydrated, and have a sober friend present
- Seek medical care immediately for chest pain, severe headache, visual changes, or extreme anxiety
Managing Agitation When Both Drugs Are Involved
In emergency settings, clinicians sometimes encounter patients with mixed stimulant and ketamine exposure.
The ACEP clinical policy on severe agitation recommends benzodiazepines as first-line treatment for stimulant-driven agitation because they counter the sympathomimetic syndrome and reduce seizure risk.
Ketamine can be used when rapid control is essential to protect patient or staff safety, but it carries risks of hypertension, tachycardia, and airway complications.
Protocols must ensure monitoring capacity and readiness to manage emergence reactions, which occur in 10 to 20 percent of cases and are typically treated with benzodiazepines.
When both drugs are suspected, clinicians should anticipate additive pressor effects and have antihypertensive medications available.
Airway equipment should be at hand due to rare but serious risks of laryngospasm or respiratory depression.

What the Evidence Shows?
No randomized, controlled human studies have directly evaluated the hemodynamic or psychiatric outcomes of simultaneous therapeutic ketamine and Adderall administration. Current risk characterization relies on:
- Mechanistic pharmacology showing overlapping catecholamine pathways
- Product labeling requiring blood pressure monitoring for esketamine
- Emergency medicine and anesthesia practice experience
- Pharmacovigilance signals for psychiatric adverse events
- Case reports of paradoxical mood worsening
This evidence base supports caution but highlights the need for dedicated clinical trials to quantify absolute and relative risks in therapeutic populations.
Practical Recommendations
For patients on Adderall considering ketamine or esketamine treatment:
- Discuss stimulant use with your ketamine provider before starting treatment
- Expect pre-dose cardiovascular screening and vital sign monitoring
- Plan to hold or reduce your Adderall dose on treatment days
- Report any chest pain, severe headache, visual changes, or worsening mood immediately
- Understand that paradoxical mood worsening, though rare, can occur and requires stopping treatment
For clinicians managing co-exposure:
- Implement standardized protocols for stimulant dose management on ketamine days
- Require pre-dose blood pressure and heart rate checks with deferral thresholds
- Monitor vital signs at baseline and intervals post-dose for at least two hours
- Screen for mood worsening and suicidality at each session
- Have antihypertensive and anxiolytic medications available
For harm reduction in recreational contexts:
- Avoid mixing stimulants and ketamine outside medical supervision
- Be aware of adulteration risks in stimulant supplies
- Use the lowest doses if co-use occurs, and have a sober friend present
- Seek emergency care for warning signs of cardiovascular or psychiatric crisis
When to Seek Help?
If you or someone you know is struggling with stimulant misuse, ketamine use, or co-occurring mental health issues, professional support can make a difference.
Integrated treatment that addresses both substance use and underlying psychiatric conditions offers the best chance for lasting recovery.
At Thoroughbred Wellness & Recovery, we provide compassionate, evidence-based care for individuals facing addiction and mental health challenges.
Our team understands the complexities of polysubstance use and co-occurring disorders, and we’re here to help you find clarity and freedom. Reach out today to learn more about Thoroughbred’s Wellness and Recovery programs.